Tuesday, October 4, 2011
Monday, October 3, 2011
Thursday, September 29, 2011
Calomel Electrode ( Mercury-Mercurous electrode)- Secondary Reference Electrode
 |
| Calomel Electrode |
Standard Hydrogen electrode is a primary reference electrode, the usage is difficult as it has many disadvantages. Other electrodes with constant electrode potential are called as secondary reference electrodes. Calomel electrode and Silver-Silver chloride electrodes are examples for such reference electrodes.
Calomel electrode cell representation is
Calomel electrode cell representation is
Hg | Hg2Cl2 (s) | KCl or Hg , Hg2Cl2 (s) , KCl.
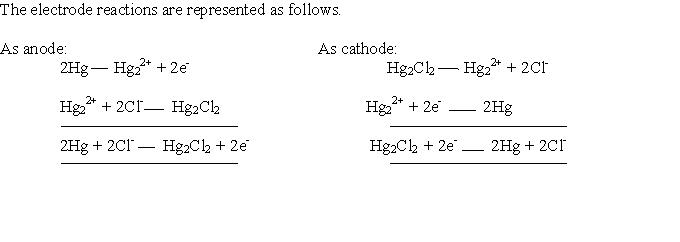
Calomel electrode acts as an anode or cathode electrode depending on the nature of the other electrode.
Tuesday, September 6, 2011
Hardness of Water
1. The hardness of water is due to the presence of
(a) Na+ and K+ (b) Na+ and Al3+
(c) K+ and Ca2+ (d) Ca2+ and Mg2+
Ans. (d)
2. Which of the following causes permanent hardness
(a) NaCl (b) MgCl2 (c) KCl (d) MgCO3
Ans. (b)
3. Which of the following causes temporary hardness
(a) NaCl (b) MgCl2 (c) KCl (d) MgCO3
Ans. (d)
4. Which of the following does not cause permanent hardness
(a) NaCl (b) MgCl2 (c) CaCl2 (d) MgSO4
Ans. (a)
(a) Na+ and K+ (b) Na+ and Al3+
(c) K+ and Ca2+ (d) Ca2+ and Mg2+
Ans. (d)
2. Which of the following causes permanent hardness
(a) NaCl (b) MgCl2 (c) KCl (d) MgCO3
Ans. (b)
3. Which of the following causes temporary hardness
(a) NaCl (b) MgCl2 (c) KCl (d) MgCO3
Ans. (d)
4. Which of the following does not cause permanent hardness
(a) NaCl (b) MgCl2 (c) CaCl2 (d) MgSO4
Ans. (a)
Subscribe to:
Posts (Atom)