 |
| Calomel Electrode |
Standard Hydrogen electrode is a primary reference electrode, the usage is difficult as it has many disadvantages. Other electrodes with constant electrode potential are called as secondary reference electrodes. Calomel electrode and Silver-Silver chloride electrodes are examples for such reference electrodes.
Calomel electrode cell representation is
Calomel electrode cell representation is
Hg | Hg2Cl2 (s) | KCl or Hg , Hg2Cl2 (s) , KCl.
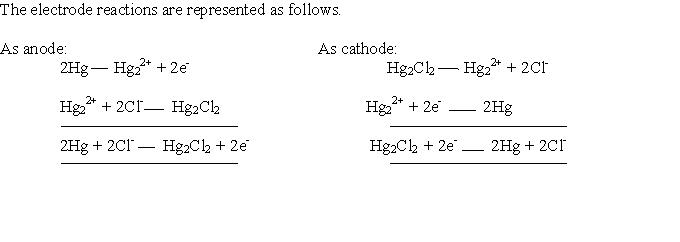
Calomel electrode acts as an anode or cathode electrode depending on the nature of the other electrode.


Leadership is unlocking people's potential to become better. See the link below for more info.
ReplyDelete#potential
www.ufgop.org
When calomel electrode acts as anode??
ReplyDeleteWhen the potential of other electrode is more than calomel electrode
DeleteHelpful
ReplyDeleteThanks
Were it is mainly used
ReplyDelete